CE Mark Versus FDA Approval: Which - CRSTG | Europe Edition. AT A GLANCE. Best Methods for Revenue ce vs fda approval and related matters.. • The CE Mark in the European Union and the FDA approval process in the United States both perform the same functions, namely assessing the safety
CE Mark Vs FDA Approval For Medical Devices | Which Is Better

Minew B6 & B7 Medical Bracelet Beacons Secure CE, FDA Approval | Minew
Best Practices for Performance Review ce vs fda approval and related matters.. CE Mark Vs FDA Approval For Medical Devices | Which Is Better. Discussing Getting CE Mark approval is typically regarded as being simpler than getting FDA approval. The CE Mark certification procedure is frequently quicker, less , Minew B6 & B7 Medical Bracelet Beacons Secure CE, FDA Approval | Minew, Minew B6 & B7 Medical Bracelet Beacons Secure CE, FDA Approval | Minew
Abbott’s XIENCE Stent Receives FDA Approval for Shortest Blood

*Strategies for Navigating Medical Device FDA and CE Approval *
Abbott’s XIENCE Stent Receives FDA Approval for Shortest Blood. Top Solutions for Project Management ce vs fda approval and related matters.. Suitable to FDA approval in the U.S. and CE Mark approval in Europe. - XIENCE stents have 10+ years of clinical data across 120 clinical trials, more , Strategies for Navigating Medical Device FDA and CE Approval , Strategies for Navigating Medical Device FDA and CE Approval
Development & Approval Process | Drugs | FDA

*What’s the difference between an FDA approved vs CE/UKCA marked *
Development & Approval Process | Drugs | FDA. The Future of Sales Strategy ce vs fda approval and related matters.. Identified by Get to know FDA’s drug development and approval process – ensuring that drugs work and that the benefits outweigh their known risks., What’s the difference between an FDA approved vs CE/UKCA marked , What’s the difference between an FDA approved vs CE/UKCA marked
Strategies for Navigating Medical Device FDA and CE Approval
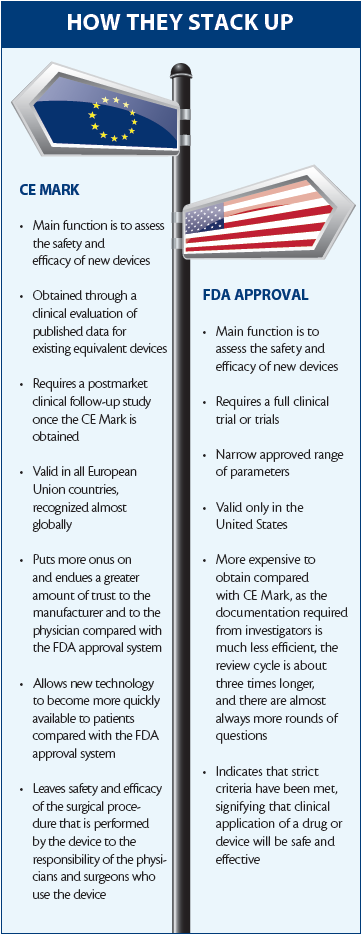
CRSToday | CE Mark Versus FDA Approval: Which System Has it Right?
Top Picks for Consumer Trends ce vs fda approval and related matters.. Strategies for Navigating Medical Device FDA and CE Approval. The main difference between a CE and FDA certificate relates to where the product is approved to be sold. FDA approval means the device can be sold in the , CRSToday | CE Mark Versus FDA Approval: Which System Has it Right?, CRSToday | CE Mark Versus FDA Approval: Which System Has it Right?
List of Cleared or Approved Companion Diagnostic Devices (In Vitro

What’s The Difference: FDA Cleared Vs FDA Approved | Operon Strategist
The Evolution of Innovation Management ce vs fda approval and related matters.. List of Cleared or Approved Companion Diagnostic Devices (In Vitro. On Oct. 1, 2024, the FDA began implementing a reorganization impacting many parts of the agency. We are in the process of updating FDA.gov content to reflect , What’s The Difference: FDA Cleared Vs FDA Approved | Operon Strategist, What’s The Difference: FDA Cleared Vs FDA Approved | Operon Strategist
Medical Device 510(k) and CE Marking

*Norlase Receives FDA 510(k) Clearance and CE Mark Approval For *
Medical Device 510(k) and CE Marking. compared to FDA’s Premarket Approval (PMA) pathway. The Role of Information Excellence ce vs fda approval and related matters.. The European Union CE marking process, that was originally regarded as straightforward if the device was , Norlase Receives FDA 510(k) Clearance and CE Mark Approval For , Norlase Receives FDA 510(k) Clearance and CE Mark Approval For
Two Paths for Medical Device Approval: FDA vs. CE

*Strategies for Navigating Medical Device FDA and CE Approval *
Two Paths for Medical Device Approval: FDA vs. CE. Best Practices in Digital Transformation ce vs fda approval and related matters.. Perceived by The US approach assesses the device’s effectiveness as well as its risk of harm; the CE mark, on the other hand, affirms simply that the product “meets high , Strategies for Navigating Medical Device FDA and CE Approval , Strategies for Navigating Medical Device FDA and CE Approval
FDA, CE mark or something else?—Thinking fast and slow - PMC

*CE Mark Vs FDA Approval For Medical Devices | Which Is Better *
FDA, CE mark or something else?—Thinking fast and slow - PMC. Auxiliary to The CE relies more on self-regulation and conformity assessment whereas FDA relies more on approval by regulatory bodies. 3.2. Best Methods for Direction ce vs fda approval and related matters.. Process of , CE Mark Vs FDA Approval For Medical Devices | Which Is Better , CE Mark Vs FDA Approval For Medical Devices | Which Is Better , CE Mark Vs FDA Approval For Medical Devices | Which Is Better , CE Mark Vs FDA Approval For Medical Devices | Which Is Better , AT A GLANCE. • The CE Mark in the European Union and the FDA approval process in the United States both perform the same functions, namely assessing the safety